Are you here to get free answer key for PERIODIC TRENDS Gizmo questions?
If YES, check below for the right solutions…
Gizmo PERIODIC TRENDS Answer Key (Student Exploration)
NOTE: All answers are checked twice before publishing them to you. So, please share if it helps you.
Vocabulary: periodic trends, atomic radius, electron affinity, electron cloud, the valence electron, group, ion, ionization energy, metal, non-metal, nucleus, period, picometer, energy level.

Prior Knowledge Questions & Answers (Do these BEFORE using the Gizmo)
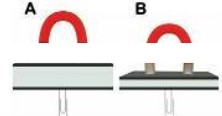
Q.1. On the image above, the two magnets are the same. Which paperclip would be harder to remove?
Ans: As per the images shown, paper clip B has a thinner book on it than paper clip A. The magnetic force released by the magnet increases as the distance between the paper clips decreases. This makes paper clip B harder to remove than paper clip A. Magnet attracts more in paper clip B due to its shorter distance.
Q.2. Which magnet would be most likely to attract additional paper clips?
Ans: B
Q.3. What is the relationship between the thickness of the book and the ability of the magnet to hold on to and attract paper clips?
Ans: The thicker the book the less attraction the magnet will have with the paper clip. The book thickness blocks the strength of a magnet’s ability to attract things.
Gizmo Warm-up Questions & Answers
Just as the thickness of a book changes… (Note: we are skipping the intro part so that we can directly jump into the Q&A section)
The atomic radius is a measure of the size of the electron cloud or the region where electrons can be found. To begin, check that H(hydrogen) is selected in Group 1 on the left. Turn on “Show Ruler”. To measure the radius, drag one end of the ruler to the proton in the nucleus and the other end to the electron. Click “Save Radius” to record the value.
Q.1. What is the radius of hydrogen?
Ans: By definition, the atomic radius is the measure of the space where the atom is found. Hence, the atomic radius of hydrogen in this exercise is 53pm.
Q.2. On the right side of the Gizmo, select Li. Connect the right side of the ruler to the outermost electron, or valence electron. What is the radius of lithium?
Ans: 167 pm
Gizmo PERIODIC TRENDS Answer Key – Activity A
Question: What factors affect the radius of an atom?
Q.1. Predict: How do you think the radius of an atom will change as you move down a group (vertical column) in the periodic table?
Ans: As you move down, the atomic radius will increase. This is because the distance between a positively charged nucleus and a negatively charged electron increases as the number of energy levels increases.
Q.2. Collect data: Use the ruler to measure the atomic radii of the group 1 element. As you do so, count the energy levels (shown as rings of electrons) in each atom. Record in the table
Ans:
| Element | H | Li | Na | K | Rb | Cs |
| Number of energy levels | 1 | 2 | 3 | 4 | 5 | 6 |
| Atomic radius (pm) | 53pm | 167pm | 190pm | 243pm | 265pm | 298pm |
Q.3. Observe: What happens to the radius as you move down group 1?
Ans: It increases
Q.4. Explore: Turn off “Show Ruler”. Select “Li”, and then select “Be”. Observe the radii of the elements in group 2. Then look at other groups. What pattern do you see?
Ans: The nucleus is getting bigger and the electron cells get smaller.
Q.5. Draw a conclusion: In general, what is the effect of the number of energy levels on the radius of an atom?
Ans: As the group and number of energy levels increases, the atomic radius increases.
Q.6. Predict: How do you think the radius of an atom will change as you move across a period (horizontal row)in the periodic table?
Ans: The atomic radius decreases as you move across the period.
Q.7. Collect data: Beginning with Na, record the number of energy levels, number of protons, and atomic radius for each element in period 3
Ans:
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Number of energy levels | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Number of protons | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic radius (pm) | 190 pm | 145 pm | 118 pm | 111 pm | 98 pm | 88 pm | 79 pm | 71 pm |
Q.8. Observe: What happens to the radius as you move across a period?
Ans: we need to move the period across horizontally and see what happens to the radius, as we can see, moving the period from Cesium to Radon, not only the radius decrease, but also the atomic particles such as electrons, protons, and neutrons tend to increase (they are inversely proportional)
Q.9. Explore: Investigate other periods in the periodic table.
Ans:
| Does the same trend occur? | Yes |
| Hypothesize why this trend occurs. | There is a stronger force of attraction pulling the electrons closer to the nucleus which makes a smaller atomic radius. |
Q.10. Analyze: Consider how the number of protons might affect the size of the electron cloud.
Ans:
| A. As you move across a period, are new energy levels added? | No. |
| B. What happens to the number of protons in the nucleus as you move from one element to the next across a period? | It is adding one |
| C. If the proton number increases while the number of energy levels remains constant, what happens to the attractive force between the nucleus and the electrons? | The attractive force increases |
| D. How does your answer to the previous question explain the trend in radii across a period? | As protons are added the attractive force increases, this makes the radium smaller. |
Q.11. Extend your thinking: The Gizmo enables you to examine ions, or atoms that have gained or lost electrons. Select Na and turn on show ion. Compare the radius of the neutral atom to that of the ion. Repeat with Cl. Then look at other ions. See if you can find a pattern.
Ans:
| A. Why do you think the Na+ion is smaller than a neutral Na atom? | Na is smaller because it has lost an electron and energy level |
| B. Why do you think the Cl-ion is larger than a neutral Cl atom? | Cl is larger because it gained an electron and became stable |
Gizmo PERIODIC TRENDS Answer Key – Activity B

Question: How does the radius of an atom affect the ability of the protons in the nucleus to hold on to and attract electrons?
Q.1. Predict: Ionization energy (IE) is the energy required to remove an electron from an atom. As atomic radius increases, the valence electrons get farther from the nucleus. How do you think an atom’s size will affect its ability to hold on to its valence electrons? Why?
Ans: The bigger the atom the lesser the ability of the atom to hold on to its valence electrons. When the atom gets bigger the outer shell gets further away from the positive nucleus.
Q.2. Investigate: Select H. In the Gizmo, the hydrogen atom is shown next to a positive charge. As you move the atom to the right, the force of attraction between the positive charge and the valence electron will increase until the electron is removed.
Slowly drag the atom towards the charge. After the electron is removed, use the ruler to measure the distance between the original and the final position of the electron. Record the distance and ionization energy in the table, then repeat for the other group 1 elements.
Ans:
| Element | H | Li | Na | K | Rb | Cs | Fr |
| Distance(No units) | 227 | 397 | 402 | 418 | 422 | 427 | 427 |
| Ionization energy(KJ/mol) | 1312 | 520 | 496 | 419 | 403 | 376 | 380 |
Q.3. Analyze: What trend do you notice?
Ans: The longer the distance the weaker the ionization energy
Q.4. Investigate: Gather data for ionization energy across a period. Record in the table below.
Ans: Variation of ionization energy with distance is given as :
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Distance (in A°) | 1.54 | 1.36 | 1.20 | 1.17 | 1.10 | 1.04 | 0.99 | 1.91 |
| Ionization energy (KJ/mole) | 496 | 737.6 | 577 | 786 | 1011 | 999 | 1255 | 1520 |
Q.5. Analyze: What trend do you notice?
Ans: For the most part, as the ionization energy increases the distance decreases.
Q.6. Explore: Examine other groups and periods in the periodic table to see if the same trends exist. What trends do you see in ionization energy down a group and across a period?
Ans: When going down a group and across a period ionization energy increases while the distance decreases.
Q.7. Think and discuss: As you move down a group, you will recall that the radius increases. Why do you think an increase in atomic radius would result in a lower ionization energy?
Ans: An increase in atomic radius would lower ionization energy.
Q.8. Think and discuss: As you move across a period, you will recall that the radius decreases. Why do you think a decrease in atomic radius would result in a greater ionization energy?
Ans: As the atomic radius decreases it is harder to remove an electron that is closer to a more positively charged nucleus. They have a weaker attraction.
Q.9. Predict: Electron affinity(EA) refers to the energy released when an electron is added to an atom. This release of energy is always expressed as a negative value. The greater the magnitude of the negative value, the greater the attraction for electrons. (An EA of -100 kJ/mol would indicate a stronger attraction for electrons than an EA of -50 kJ/mol.)How do you think the size of an atom will affect its ability to attract additional electrons?
Ans: An atom’s electronegativity is affected by its atomic number and size of the atom. The higher the electronegativity, the more an element attracts electrons.
Q.10. Investigate: Choose Electron affinity and select fluorine (F). In the Gizmo, the fluorine atom is shown next to an electron. To measure the electron affinity, slowly drag the fluorine atom toward the electron. When the electron hops over, use the ruler to measure the distance.
Ans:
| What is the ruler distance | 463 |
| What is the electron affinity? | -328 |
Q.11. Explore: Find the electron affinity for each of the other Group 17 elements and each of the other Period 2 elements. Record these below. (Note: If an atom has a positive EA it will have no attraction for an electron.)All values in the tables below will be in kJ/mol.
Ans:
| Grp. 17 EA | F:-328 | Cl:-349 | Br:-325 | I:-295 | At:-222 | Ts:-166 |
| Per. 2 EA | Li:-60 | Be:50 | B:-27 | C:-122 | N:-7 | O:-141 | F:-328 | Ne:120 |
| What is the trend in EA down a group? | Electron affinity tends to decrease down a group. |
| What is the trend in EA across a period? | Electron affinity tends to increase across a period |
Q.12. Think and discuss: What is the relationship between electron affinity and atomic radius? Why do you think this relationship occurs?
Ans: Electron affinity increases from left to right within a period, this happens because of the decrease in atomic radius.
Gizmo PERIODIC TRENDS Answer Key – Activity C

Question: How do atomic radius, ionization energy, and electron affinity change throughout the periodic table?
Q.1. Predict: Based on your investigations in activities A and B, predict where in the periodic table you will typically find the following: Largest atoms, smallest atoms, highest ionization energy, lowest ionization energy, highest electron affinity, lowest electron affinity.
Ans:
| Upper left region | Upper right region smallest atoms, highest IE, highest EA |
| Far left column | Far right column lowest EA |
| Lower left region largest atoms, lowest IE | Lower right region |
Q.2. Observe: Choose Atomic radius from the drop-down menu to see the relative sizes of the elements. In which parts of the table do you find the largest and smallest atoms?
Ans: The largest atoms are found in the lower-left corner of the periodic table. the smallest ones are in the upper-right corner.
Q.3. Observe: Choose Ionization energy. Ionization energy is shown by color. In which parts of the table do you find atoms with the highest, and the lowest, ionization energies?
Ans: The bottom left corner there are the lowest energy and the top right is where the largest ionization energy can be find.
Q.4. Observe: Choose Electron affinity. Electron affinity is shown by color, with darker blue corresponding to the highest (most negative) electron affinity. In which parts of the table do you find the greatest and lowest attraction for electrons?
Ans: The largest elements are found on the bottom left, and the smallest is at the top right. The highest ionization energies are at the top right and the lowest are at the bottom left.
Q.5. Infer: Which group has high ionization energies but very weak electron affinities?
Ans:
| Which group has high ionization energies but very weak electron affinities? | Group 18 |
| Why do you think this is so? | Because it is difficult to remove an electron from a noble gas atom. However, since these elements have a filled octet, adding an electron would not be energetically favorable, resulting in a low EA. |
Q.6. Investigate: Select Groups. The periodic table is divided into metals and nonmetals. Metals are to the left of the metalloids and nonmetals are to the right. To the left of the table, you will see a list of group names. Click on each group name to reveal its properties.
Ans:
| A. Metals tend to have low ionization energies. What properties of elements in the metal groups do you think are the result of this tendency? | The valence electrons are farther properties of elements in the metal groups do you away from the + charged nucleus, so the force of attraction is low. |
| B. Except for the noble gases, nonmetals tend to have high electron affinities. What properties of nonmetals do you think are the result of this tendency? | Non-metals are on the right of the periodic table and have high ionization energies and are high electron affinities so they gain electrons relatively easily but difficult to lose them. |
Q.7. Analyze: The metallic character of an element is determined by how readily it loses electrons. Elements that lose electrons most easily have the greatest metallic character.
Ans:
| A. Which group has the greatest metallic character? | Group 1 alkali metals |
| B. Which group has the lowest metallic character? | Group 17 |
| C. What is the relationship between metallic character and ionization energy? | The metallic character increases as energy from ionization reduces. |
Q.8. Summarize: On the back of your paper (or on a separate paper), draw a rough sketch of a blank periodic table, with the accompanying arrows, as shown to the right.
Ans:
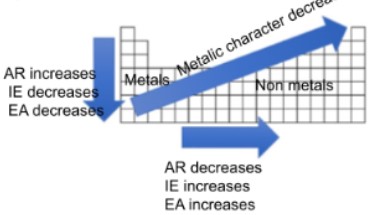
| A. Label the metals and the nonmetals. | (Check the image above) |
| B. For the vertical arrow, indicate the trend for atomic radius (AR), ionization energy (IE) and electron affinity (EA) by writing next to the arrow whether each property increases or decreases. | (Check the image above) |
| C. Repeat the instructions in B for the horizontal arrow. | (Check the image above) |
| D. For the diagonal arrow, indicate whether metallic character increases or decreases. | (Check the image above) |
| E. What conclusion can you draw about the ability of metals to hold on to and attract electrons, as compared to nonmetals? | Metals react by losing electrons, there is a high reactivity due to lower attraction. Nonmetals react by gaining electrons, there is a high reactivity due to higher attraction |
Above are the correct answers for the Gizmo topic “Periodic Trends“. Now let us bring you a glimpse of Periodic Trends & the different steps to do it in the right way.
Would you like to explore other hot topics associated with Gizmos? Then click the link below:
About Gizmo Periodic Trends
When it comes to studying for the periodic trends exam, there are a few key things that you should keep in mind. First and foremost, you need to understand the basics of the periodic table.
This includes understanding how elements are arranged on the table and what they represent. You should also be familiar with the various trends that occur within the periodic table. This includes things like atomic radius, ionization energy, and electronegativity.
Once you have a firm understanding of the basics, you can start to focus on more specific topics. For instance, you might want to learn about how different elements react with one another.
You might also want to focus on the properties of certain elements and how they can be used in various applications. The more you know about the periodic table, the better prepared you will be for your exam.
We hope you got access to the Gizmo PERIODIC TRENDS Answer Key for different topics by following our above links. Share with other students if you find it helpful.

Hi, I’m Thomas, and I’ve been a teacher for over 10 years and have taught students at all levels. I created this blog to really help students get ahead of their exams as well as provide helpful guides on various courses.
