Are you here to get FREE answer key for Balancing Chemical Equations Gizmo questions?
If YES, check below for the right solutions…
Balancing Chemical Equations Gizmo Answer Key [FREE ACCESS]
NOTE: All answers are checked twice before publishing them to you. So, please share if it helps you.
Vocabulary: coefficient, combustion, compound, decomposition, double replacement, element, molecule, product, reactant, single replacement, subscript, synthesis

Prior Knowledge Questions & Answers
Introduction: The scouts are making s’mores out of toasted marshmallows, chocolate, and graham crackers.
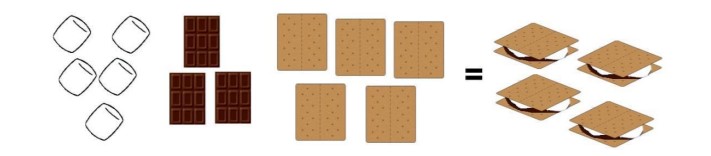
Q.1. What is wrong with the above image?
Ans: All together it equals 4 smores however the quantity of the marshmallows, chocolate, and gram crackers are not the same. Despite there only being 3 chocolate bars, 4 smores were made. Also, 4 smores would require 8 graham crackers but there are only 5 crackers shown.
Q.2. Assuming a s’more requires two graham crackers, one marshmallow, and one piece of chocolate, how many smores could you make with the ingredients shown?
Ans: With this assumption and the ingredients shown, only 2 full smores could be made.
Gizmo Warm-up Questions & Answers
In a chemical reaction, reactants interact to form products. This process is summarized by a chemical equation. In the Balancing Chemical Equations Gizmo, look at the floating molecules below the initial reaction: H2 + O2 🡪 H2O.
Q.1. How many atoms are in a hydrogen molecule (H2)?
Ans: 2
Q.2. How many atoms are in an oxygen molecule (O2)?
Ans: 2
Q.3. How many hydrogen and oxygen atoms are in a water molecule (H2O)?
Ans: 1 Oxygen & 2 Hydrogen
Q.4. In general, what does a subscript (such as the “2” in H2) tell you about the molecule?
Ans: The subscript means the number of atoms of each element is in the molecule.
Q.5. A chemical equation is balanced if the number of each type of atom on the left side is equal to the number of each type on the right side. Is this reaction balanced?
Ans: No, because there are two oxygen atoms on the reactant’s side of the equation and only one product side of the equation.
Balancing Chemical Equations Gizmo Answers – Activity A

Introduction: The equation H2 + O2 🡪 H2O is unbalanced because there are two oxygen atoms on the reactant’s side of the equation and only one on the product’s side of the equation. To balance the equation, you cannot change the structure of any of the molecules, but you can change the number of molecules that are used.
Question: How are chemical equations balanced?
Q.1. Balance: Turn on Show histograms. The equation is balanced when there are equal numbers of each type of atom represented on each side of the equation. In the Gizmo, use the up and down arrows to adjust the numbers of hydrogen, oxygen, and water molecules until the equation is balanced. When you are done, turn on Show summary to check your answer. Write the balanced equation here:
Ans: 2 H2 + 1 O2 🡪 2 H2O
Q.2. Solve: Turn off the Show summary. Use the Choose reaction drop-down menu to see other equations, and balance them. Check your answers and then write the balanced equations:
Ans:
2 Al + 6 HCl 🡪 2 AlCl3 + 3 H2
2 NaCl 🡪 2 Na + 1 Cl2
1 Na2S + 2 HCl 🡪 2 NaCl + 1 H2S
1 CH4 + 2 O2 🡪 1 CO2 + 2 H2O
Q.3. Practice: Balance the following chemical equations. (These equations are not in the Gizmo.)
Ans:
A. 2 Na + 1 Cl2 🡪 2 NaCl
B. 2 Na + 2 H2O 🡪 2 NaOH + 1 H2
C. 2 Mg + 1 O2 🡪 2 MgO
D. 2 KClO3 🡪 2 KCl + 3 O2
E. 2 Al + 3 CuO 🡪 1 Al2O3 + 3 Cu
F. 1 I2 + 2 Na2S2O3 🡪 2 NaI + 1 Na2S4O6
G. 6 Mg + 1 P4 🡪 2 Mg3P2
Balancing Chemical Equations Gizmo Answers – Activity B

Introduction: Chemical equations show how compounds and elements react with one another. An element is a substance consisting of one kind of atoms, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atoms, such as water (H2O) or table salt (NaCl).
Question: How are chemical reactions classified?
Q.1. Match: Most chemical reactions can be classified as one of four types. Using the chemical equations in the gizmo as a guide, match the following definitions to the type of reaction:
Ans:
A-One reactant is broken down into two or more products. A. Synthesis
E-A fuel is combined with oxygen to produce carbon dioxide and water. B. Decomposition
C-Two or more reactants combine to form one product. C. Single replacement
D-Two compounds react to form two different compounds. D. Double replacement
B- A compound reacts with an element to form a new compound & a different element. E. Combustion
Q.2. Practice: Balance each of the chemical equations below. (Some equations may already be in balance.) In the space to the right, classify the reaction as a synthesis, decomposition, single replacement, or double replacement reaction.
Ans:
A. Already Balanced AgNO3 + Double Displacement KCl 🡪 AgCl + KNO3
B. Already Balanced H2O + Synthesis SO3 🡪 H2SO4
C. 2 KI + 1 Single Displacement Cl2 🡪 2 KCl + I2
D. 2 NaHCO3 🡪 1 Decomposition Na2CO3 + 1 H2O + 1 CO2
E. 1 Zn + 2 Single Displacement HCl 🡪 1 ZnCl2 + 1 H2
F. 1 BaCl2 + 1 Double Displacement Na2SO4 🡪 1 BaSO4 + 2 NaCl
G. 1 C3H8 + 5 Combustion O2 🡪 3 CO2 + 4 H2O
H. 2Al + 3 single displacement CuCl2 🡪 2 AlCl3 + 3 Cu
Above are the correct answers for the Gizmo topic “Balancing Chemical Equations“. Now let us bring you a glimpse about Balancing Chemical Equations & the different steps to do it in the right way.
Or would you like to explore other topics associated with Gizmos? Then >>Check Other Gizmo Answers<<
About Balancing Chemical Equations
Balancing chemical equations may seem like a difficult task, but it is actually quite simple. All you will need is some basic math and knowledge of the laws of chemistry.
The first thing that you should do when balancing chemical equations is write down the unbalanced equation in front of you, and then identify whether it is a synthesis or decomposition reaction. Once you have decided this, all you will need to do is follow a simple formula to balance the equation.
The simplest way to balance a chemical equation is by using the law of conservation of mass and balancing the atoms on both sides of the equation.
This means that you need to identify and count all of the atoms on both sides of the reaction. If you do this and they are the same, then you can cross out all of those individual atoms and you will have a balanced equation.
Steps to Balance a Chemical Equation
Balancing chemical equations can be done in many ways, including coefficients, variables, and reduction-oxidation numbers. Once it is balanced, there will always be as many molecules on the right side as there were before all the equations were balanced.
- The first step is to identify all the reactants and products in the equation. The reactants are listed on the left side of an arrow, or in roman numerals, while the products are written on the right side of an arrow.
- Once it is clear what all elements are doing in a reaction, one needs to balance each element in order to have equal numbers on both sides of the arrow. The coefficients in front of each element are how many atoms of that specific element are present.
- If the equation has a coefficient that is a fraction, then it can be reduced to a smaller fraction with a common denominator or multiplied by a number to make it an integer. When there are fractions next to each other with different denominators, they can be combined using the least common multiple of the two denominators.
- When there is a coefficient in front of an arrow, it means “yields.” This simply means that when one atom is changed to ions and molecules, it creates more than one product for every product on the right side. For example, when hydrogen ions are changed to hydrogen gas, it yields two hydrogen atoms.
- Another way to change an equation is by eliminating coefficients completely. For example, if there is one atom on the left and four on the right, then one would multiply both sides by five to eliminate the coefficient. It could also be done with variables, like in the case when one atom is on each side. When this occurs, x can be multiplied by five to make it y.
- Last but not least, when balancing equations there are times when reduction and oxidation numbers need to be accounted for. Reduction is done when hydrogen ions gain electrons while oxidation is done when an atom gains electrons. An oxidation reaction causes the substance being multiplied to have a positive number, while a reduction reaction causes it to have a negative one. The oxidation numbers must be equal on both sides of the equation and the reduction numbers must be equal as well.
- After balancing a chemical equation is complete, there may be more ions than atoms in a final product. When this occurs, the equation must be balanced again. This time it is done with coefficients that have a negative in front of them. The final product will have as many atoms as there were before all the equations were balanced.
In conclusion, chemical equations need to be balanced in order to make sure each element has an equal number of atoms on the left and right sides of an arrow.
More References:
=> https://study.com/academy/lesson/balanced-chemical-equation-definition-examples.html
That’s all. Hope you find the Balancing Chemical Equations Gizmo Answer Key for levels A & B, a detailed summary by following our answers above.
Share with your batchmates if you find it helpful.

Hi, I’m Thomas, and I’ve been a teacher for over 10 years and have taught students at all levels. I created this blog to really help students get ahead of their exams as well as provide helpful guides on various courses.

i <3 u