Are you here to get FREE answers key for Ionic Bonds Gizmo questions?
If YES, check below for the right solutions…
Student Exploration Ionic Bonds Gizmo Answer Key
NOTE: All answers are checked twice before publishing them to you. So, please share if it helps you.
Vocabulary: chemical family, electron affinity, ion, ionic bond, metal, nonmetal, octet rule, shell,
the valence electron, heavy metal

Prior Knowledge Questions
Q.1. Nate and Clara are drawing pictures with markers. There are 8 markers in a set. Nate has 9
markers and Clara have 7. What can Nate and Clara do so that each of them has a full set?
Ans: Nate can give a marker to Clara
Q.2. Maggie is sitting at a table with Fred and Florence. Maggie has 10 markers, but Fred and
Florence each has only 7 markers. How can they share markers so each has 8?
Ans: Maggie can give each guy one marker so that they all have 8 markers
Gizmo Warm-Up Questions & Answers
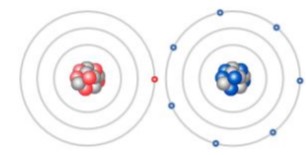
Just like students sharing markers, atoms sometimes share or swap electrons. By doing this,
atoms form bonds. The Ionic Bonds Gizmo allows you to explore how ionic bonds form.
To begin, check that Sodium (Na) and Chlorine (Cl) are selected from the menus at the right. Click Play ( ) to see electrons orbiting the nucleus of each atom. (Note: These atom models are simplified and not meant to be realistic.)
Q.1. Each atom consists of a central nucleus and several shells that contain electrons. The outermost electrons are called valence electrons. (Inner electrons are not shown.) How many valence electrons does each atom have?
Ans: Sodium:1 Chlorine:7
Q.2. Click Pause ( ). Elements can be classified as metals and nonmetals. Metals do not hold on to their valence electrons very tightly, while nonmetals hold their electrons tightly. Electron affinity is a measure of how tightly the valence electrons are held.
A. Try pulling an electron away from each atom. Based on this experiment, which atom is a metal?… Ans: Sodium …Which is a nonmetal?… Ans: Chlorine
B. Try moving an electron from the metal to the nonmetal. What happens?… Ans: The metal loses its outside ring and the non-metal accepts the electron on the outermost ring.
Ionic Bonds Gizmo Answer Key – Activity A

Introduction: Some of the particles that make up atoms have an electrical charge. Electrons are negatively charged, while protons are positively charged. Particles with opposite charges (+ and –) attract, while particles with the same charge (+ and + or – and –) repel.
Question: What happens when atoms gain or lose electrons?
Q.1. Count: Electrons move around the nucleus of atoms in specific shells, shown by the rings
around the atoms in the Gizmo. The first ring holds two electrons, and the second holds eight. (Electrons in the inner rings are not shown; you can assume these rings are full.)
A. Observe the sodium and chlorine atoms. Assuming that the inner rings are full of electrons, how many electrons are there total in each atom?… Ans: Sodium____2____ Chlorine_____10____
B. Each atom is neutrally charged, which means that each atom has the same number of protons and electrons. Based on this, how many protons are in each atom?… Ans: Sodium___3___ Chlorine___9__
Q.2. Observe: Most atoms are stable with a configuration of eight valence electrons. This is known as the octet rule. How many valence electrons does each atom have?
Ans: Sodium____1____ Chlorine___7___
Q.3. Form a bond: Each electron has a charge of 1–, and each proton has a charge of 1+. You can calculate the charge of an atom by subtracting the number of electrons from the number of protons. Move an electron from the sodium to the chlorine atom.
A. What are the charges of each atom now?… Ans: Sodium__1+_ Chlorine__1-_
B. Is each ion stable? Explain… Ans: Yes, because of the octet rule there are now 8 in the
set. It’s a sodium chloride combo.
Q.4. Think and discuss: Why is there an attraction between the two ions in this chemical bond?
Ans: Because one has a positive charge and the other has a negative charge
Ionic Bonds Gizmo Answer Key – Activity B

Question: How are ionic compounds formed?
Q.1. Observe: Look at the red lithium atom and the blue oxygen atom. Recall that most atoms are stable when their outermost ring has eight electrons. (Some atoms, such as lithium and beryllium, are stable when their outermost ring has two electrons.)
A. How many electrons will the lithium atom give up to become stable?… Ans: 1
B. How many electrons does the oxygen atom need to become stable?… Ans: 2
C. Can a stable compound be made from these two atoms? Explain why or why not… Ans: No, because oxygen needs one more electron than lithium has to give.
Q.2. Form bonds: Click Add metal to add another lithium atom, and then transfer electrons from the lithium to the oxygen. Click Check.
A. Did you make a stable compound?… Ans: Yes
B. Turn on Show formula. What is the formula of this compound?… Ans: Li2O
C. Turn on Show charge. What is the charge of each ion?… Ans: Li 1+_ Li _1+_ O _2-
Q.3. Practice: Use the Gizmo to create stable compounds from the combinations given below.
After transferring electrons, arrange the atoms to demonstrate the attraction between
positively charged ions and negatively charged ions. Click Check to check each compound.
For each compound, click the camera ( ) to take a snapshot. Paste each image into a
blank document to turn in with this worksheet. Write the ionic charges (such as Ca2+) and
chemical formulas below.
Ans:
| Ionic Charges | Chemical Formula | |
| A. Lithium & Fluorine | Li 1+ F -1 | Lif |
| B. Beryllium & Oxygen | Be 2+ O 2- | BeO |
| C. Magnesium & Fluorine | Mg 2+ F 1- | MgF2 |
| D. Aluminum & Chlorine | Al 3+ Cl 1- | AlCl3 |
| E. Beryllium & Nitrogen | Be 2+ N 3- | Be3N2 |
Ionic Bonds Gizmo Answer Key – Activity C

Introduction: The periodic table arranges elements by size and property. The vertical columns
represent chemical families or groups of elements with similar chemical properties.
Question: How are elements arranged into chemical families?
Q.1. Observe: Drag the nonmetal into the trash ( ) so there is only one lithium atom visible.
A. How many valence electrons does lithium have?… Ans: 1
B. Now look at your periodic table. Find lithium (Li) in the first column. Other than lithium, which element from the Gizmo is also in this column?… Ans: Sodium
C. Choose this element. How many valence electrons does this element have?… Ans: 1
Q.2. Gather data: Four other pairs of elements in the same chemical family are listed below. List
the number of valence electrons in each element.
Ans:
| Beryllium | 2 | Nitrogen | 5 | Oxygen | 6 | Fluorine | 7 |
| Magnesium | 2 | Phosphorus | 5 | Sulfur | 6 | Chlorine | 7 |
Q.3. Analyze: What pattern do you see?
Ans: The elements from the same family have the same number of valence electrons.
Q.4. Make a rule: Based on your data, how are elements arranged into chemical families?
Ans: By number of valence electrons
Q.5. Infer: Look at your periodic table. How many valence electrons would you find for elements
in each family?
Ans: Boron family 13 Carbon family 14 Neon family 18
Q.6. Think and discuss: How do you think the number of valence electrons relates to an
element’s chemical properties?
Ans: Maybe metals have fewer valence electrons than non-metals.
Above are the correct answers for the Gizmo (student exploration) topic “Ionic Bonds“. Now let us bring you a glimpse of the Ionic Bonds & their different processes in the coming session.
Or would you like to explore other topics associated with Gizmos? Then >>CHECK HERE <<
About Ionic Bonds
Ionic bonds are created between two ions. An ion is an atom or molecule with a net electric charge because of the loss or gain of one or more electrons.
It is this attraction between the positive and negative ions that hold the substance together, in a crystalline form – not covalent bonds as previously believed.
Cations and anions are formed when atoms lose or gain electrons, turning them into an ion.
For example, sodium (Na) is a metal that easily gives up one electron from its shell to form a cation with no charge – this is why sodium is often found in nature as Na+ . Chlorine (Cl), on the other hand, readily accepts an electron to form an anion with a negative charge – this is why chlorine tends to exist as Cl-
Ionic bonds are formed by either the attraction between cations and anions or the mutual repulsion between two cations. For example, sodium and chlorine atoms are held together by the attraction between their respective cations.
These two oppositely charged ions attract each other, forming a bond and releasing energy in the form of heat and light (when they first come into contact).
This is also what causes lightning; as clouds rub against each other during a storm, electrons are transferred from one atom to another, causing air molecules in between the clouds to become charged.
As the Earth becomes more negatively charged than the atoms in the cloud, it becomes attracted to them and is repelled by other parts of the cloud which are similarly charged. Eventually, this can cause a bolt of lightning – when all of these opposing charges meet at once.
Ionic bonds are also formed by the mutual repulsion between two cations. For example, a magnesium atom (Mg) is held together with a chlorine atom because the magnesium has two electrons in its valence shell and needs eight to become stable.
Chlorine has seven electrons in its outermost electron shell and readily accepts two more to become stable. The magnesium atom gives up its two electrons to the chlorine, forming Mg++ and Cl- .
This is what causes salt (NaCl) crystals to form; as positively charged sodium cations are repelled by other similarly charged anions in their immediate vicinity, they are drawn to similarly charged ions in neighboring molecules, forming crystalline bonds with them.
This type of bond does not happen when water molecules are involved, because the oxygen in one water molecule attracts the hydrogen atoms in another, preventing an ionic bond from forming between them.
Ionic bonds are stronger than covalent bonds – which is why substances containing ionic bonds tend to be solid in room.
More References:
=> https://www.britannica.com/science/ionic-bond
=> https://www.sciencedirect.com/topics/chemistry/ionic-bond
Hope you find Ionic Bonds Gizmo Answer Key for levels A, B & C (student exploration) by following our answers above.
Also, share with your batchmates if you find it helpful.

Hi, I’m Thomas, and I’ve been a teacher for over 10 years and have taught students at all levels. I created this blog to really help students get ahead of their exams as well as provide helpful guides on various courses.

Nice work